The primary goal of this research was to elucidate the key influences of the alloying elements in Ni-Cr-Mo alloys on their electrochemical behaviour. Two alloys, varying in Cr and Mo content, were studied in deaerated 5 M NaCl solutions.
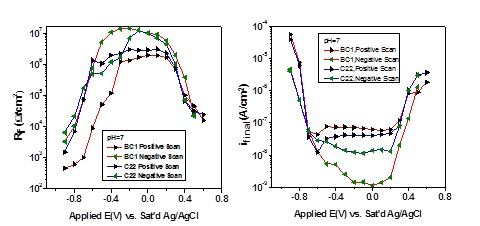
As indicated in Figure 1, potentiostatic polarization followed by electrochemical impedance spectroscopy (EIS) (to measure the passive film resistance) shows that, while the data obtained on C22 (22Cr:13Mo:3W) and BC1 (15Cr:22Mo) alloys are similar, the current density in the passive region (-0.5 – 0.2V) was slightly higher for the low Cr-containing BC1 alloy in the scan from negative to positive potentials. On the other hand, on the reverse scan, the BC1 alloy shows a much lower current and much higher film resistance.
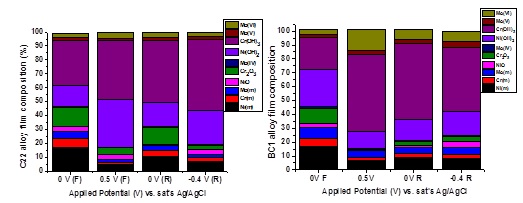
To study the film composition in forward and reverse scan, XPS and AES were used. Figure 2 shows the film compositions obtained from XPS analyses. For both alloys, increasing the potential from 0 V (passive) to 0.6 V (transpassive) (forward scan) led to an increase in film thickness, a decrease in Cr oxide content, and an increase in Mo oxide content. When the potential is subsequently reduced (reverse scan), alloy C22 reforms Cr2O3 more readily than alloy BC1. Consequently, the higher resistance of the film and lower passive current (reverse scan, Figure 1) on this alloy can be attributed to the higher Mo content of the oxide film.
By Nafis Ebrahimi, Department of Chemistry, Western University

