Recent research is looking to determine the effects of radiolytically produced HNO3 droplets on the corrosion of copper coatings on high level nuclear waste containers. The extent and distribution of corrosion damage has been analyzed to help determine a penetration rate and the reaction rate constants required to model the corrosion process.
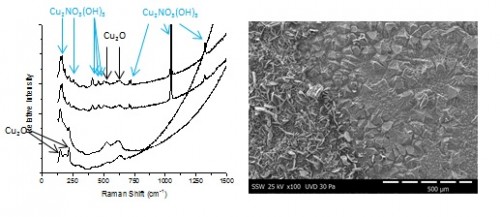
A micro-electrochemical cell is being used to monitor the evolution of solution pH in the presence of a corroding copper electrode. Various distinct regions are observed, each demonstrating different corrosion morphologies. For [HNO3] ≥ 50 mM and long exposure times, nitrate is incorporated into the oxide as rouaite (Cu2NO3(OH)3) crystals, shown in the SEM images in Figure 1. The identity of the underlying oxide (Cu2O) and the rouaite crystals was determined by Raman spectroscopy, Figure 1.
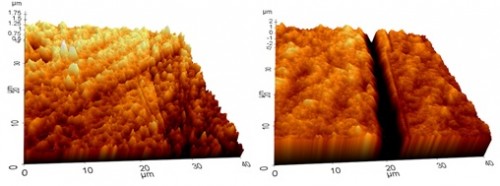
For short immersion times, significant grain boundary etching was observed with the depth of the etching of various grain boundaries being measured by AFM. Figure 2 demonstrates the increase in grain boundary etching from 3 to 10 days of immersion. The maximum depth of etching increased from ~ 0.5 µm to ~ 3.5 µm from 3 to 10 days of immersion in 100 mM HNO3.
By Joseph Turnbull, Department of Chemistry

