This recent research project was designed to investigate the influence of carbonate/bicarbonate, the key groundwater constituents likely to influence fuel dissolution inside a failed nuclear waste disposal container, on the reactivity of different uranium oxide (UO2) specimens.
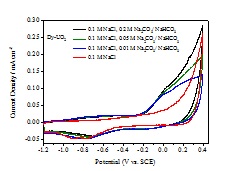
A cyclic voltammetric (CV) study of doped UO2 (Dy-UO2, Gd-UO2, 1.5 at% SIMFUEL and UO2.002) in 0.1 M NaCl with and without carbonate/bicarbonate shows the anodic current for the four UO2 electrodes is increased in the potential range -1.2 V to -0.4 V by adding carbonate/bicarbonate. The oxidation is enhanced without any detectable fuel dissolution, confirming that it is anodic oxidation of the UO2 matrix that is being enhanced.
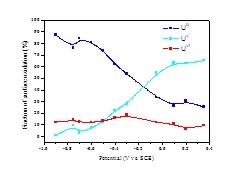
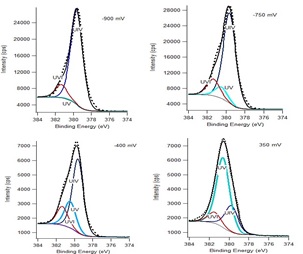
To investigate this surface oxidation mechanism, an X-ray photoelectron spectroscopic analysis of the electrochemically oxidized surface was conducted. The U 4f7/2 peak and its deconvolution into contributions from U(IV), U(V) and U(VI) is shown in Figure 3, and the change in composition with applied potential in Figure 2. Between -0.9 V and -0.4 V there is a distinguishable conversion of U(IV) to U(V) (Figure 2) as indicated in the CV scans in Figure 1.
By combining electrochemical studies with XPS analysis of Dy-UO2, a possible anodic oxidation mechanism in the potential range -1.2 V to -0.4 V is proposed. First, carbonate adsorbs on the UO2 surface to form (U(IV)O2)(CO3)y-2y, which is not quite stable, since the surface interaction involves only physisorption. This physisorbed intermediate (U(IV)O2)(CO3)y-2y is stabilized by oxidation to (U(V)O2)(CO3)y1-2y, a process that would also require the simultaneous incorporation of an O interstitial (OI) into the surface of the oxide.
By Nazhen Liu, Department of Chemistry, Western University

