Request for analysis:
Many ore deposits have varying degrees of primary and/or secondary copper minerals, the presence of which can strongly affect the flotation behaviour of the ore. The ToF-SIMS technology will be used as a predictive tool in providing an estimate of the ore capacity to undergo “inadvertent Cu activation” during the grinding process. This information will complement that gained through automated mineralogy and help complete the information package needed to better guide the metallurgist in the pre-selection of a suitable flotation process.
Objectives:
Methodology:
To establish the database of surface chemical signatures for different minerals and ore deposits, a standard protocol for sample preparation and subsequent surface analysis was developed. The test protocol includes grinding of a small amount of a feed ore sample under standardized conditions. A two-chamber ball mill was specifically designed, commissioned and tested to determine the surface reactivity of ore minerals under specified conditions. The surface modification (Cu activation) of specific mineral phases placed in a separate chamber in the ball mill (where they interact with the pulp only) is characterized by ToF-SIMS surface analysis. The development of a database (library) with Cu-bearing poly-metallic ores using this test has shown the measured concentration of Cu transfer on mineral surfaces extends over several orders of magnitude.
Results of the study:
The test data in Figure 1 represent a summary of chemical reactivity tests carried out ore samples with varying mineralogies in order to investigate the relative degree of Cu transfer from the ore minerals to the specimen pyrite surface during the grinding process.
The established chemical reactivity for ore samples #1 and #2 is compared with library data set (ores #3 #13). For comparative analyses, a baseline of Cu transfer was established by performing a number of tests under standard operating conditions (SOP) with pyrite and sand.
The variability of Cu on pyrite for the entire ore data set illustrates that the Cu content on mineral surfaces extends over several orders of magnitude dynamic range. It should be noted that some of the highest Cu loadings occurred when “non-Cu-bearing” ores were milled. For example, ore samples #1 and #2 are from the same mine, but exhibit significantly different flotation behaviour (see discussion below).
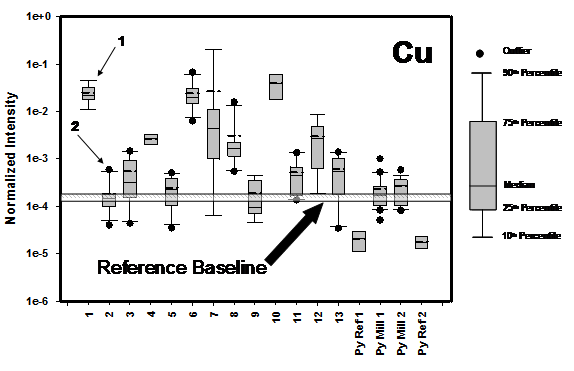
Figure 1: Vertical box plots showing ToF-SIMS data for Cu as measured on the surface of pyrite specimen grains from the chemical reactivity test for 13 different ore specimens. The data for the Py Ref represents the normalized Cu intensity on the “as received” pyrite reference samples, and for the Py mill samples for Cu on the surface of the pyrite after milling with sand. The horizontal line through the plot is the estimated baseline for the testing program. Ore numbers 1 and 2 are indicated by the arrows.
The following is a discussion regarding observations made regarding Cu transfer in relation to the mineralogical make-up of the tested ore samples. Figure 2 shows the variability of Cu surface loading on pyrite for various mineralogical zones in a single deposit. The samples show a distinct dependence of Cu loading with respect to ore type, increasing Cu content on pyrite surfaces in the following sample sequence: skarn, hypogene, supergene. This trend correlates quite well with the increase in secondary minerals in the hypogene and supergene samples (Figure 2 inset), whose solubility is greater than the primary minerals found in the skarn. The effect is an increase in solution Cu during the grind resulting in a transfer to the surface of the pyrite. The difference between the copper loading from the skarn and hypogene samples may also be related to different pH levels developed in the mill in response to the different mineralogy of the samples.
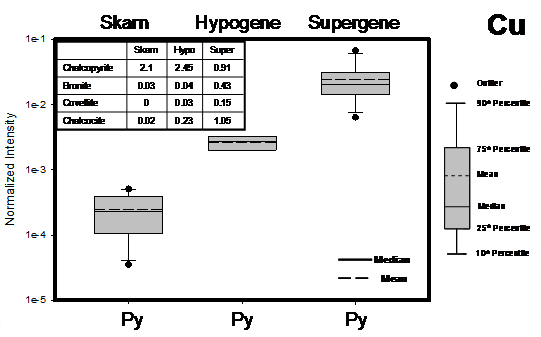
Figure 2: Skarn, hypogene, supergene ore types (ores #5, #4 and ore #6 in Figure 1): vertical box plots showing ToF-SIMS data for Cu as measured on the surface of pyrite (Py) specimen grains from the chemical reactivity test.
Major findings:
As noted above, there is a significant difference in Cu loading on the pyrite surfaces between ore samples #1 and #2 (Figure 1 and 3). Ore #1 (Figure 3) contains minimal copper mineralization (~0.05%Cu), however, in practice, this ore exhibited strong inadvertent sphalerite flotation and metallurgical testing elsewhere indicated that copper activation was indeed playing a role. By comparison, ore #2 from the same deposit with similar copper mineralization (0.05% Cu) yielded excellent flotation separation; no inadvertent sphalerite activation was noted. The flotation behaviour of ore #1 suggests that the presence of minute amounts of highly soluble copper minerals (insufficient, perhaps, to be detected in the QEMSCAN analysis) can have a substantial effect on flotation chemistry. The observed flotation response would not have been predicted from mineralogical analysis alone, and demonstrates the value of conducting this test in parallel with automated mineralogy as a preliminary ore assessment tool ahead of flotation testing.
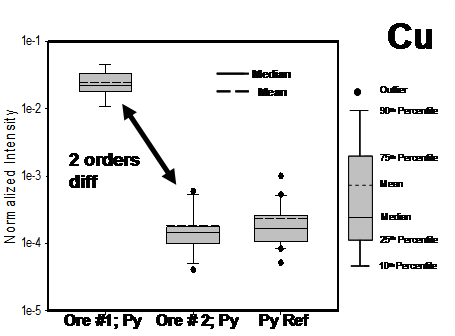
Figure 3: Ore #1 and #2 from Figure 1: vertical box plots showing ToF-SIMS data for Cu as measured on the surface of pyrite (Py) specimen grains from the chemical reactivity test. Also included is the pyrite reference sample.

